Investment
Investment Summary
Magellan Stem Cells aims to be the world first and leading supplier of off-the-shelf allogeneic stem cell therapy in the treatment of Osteoarthritis
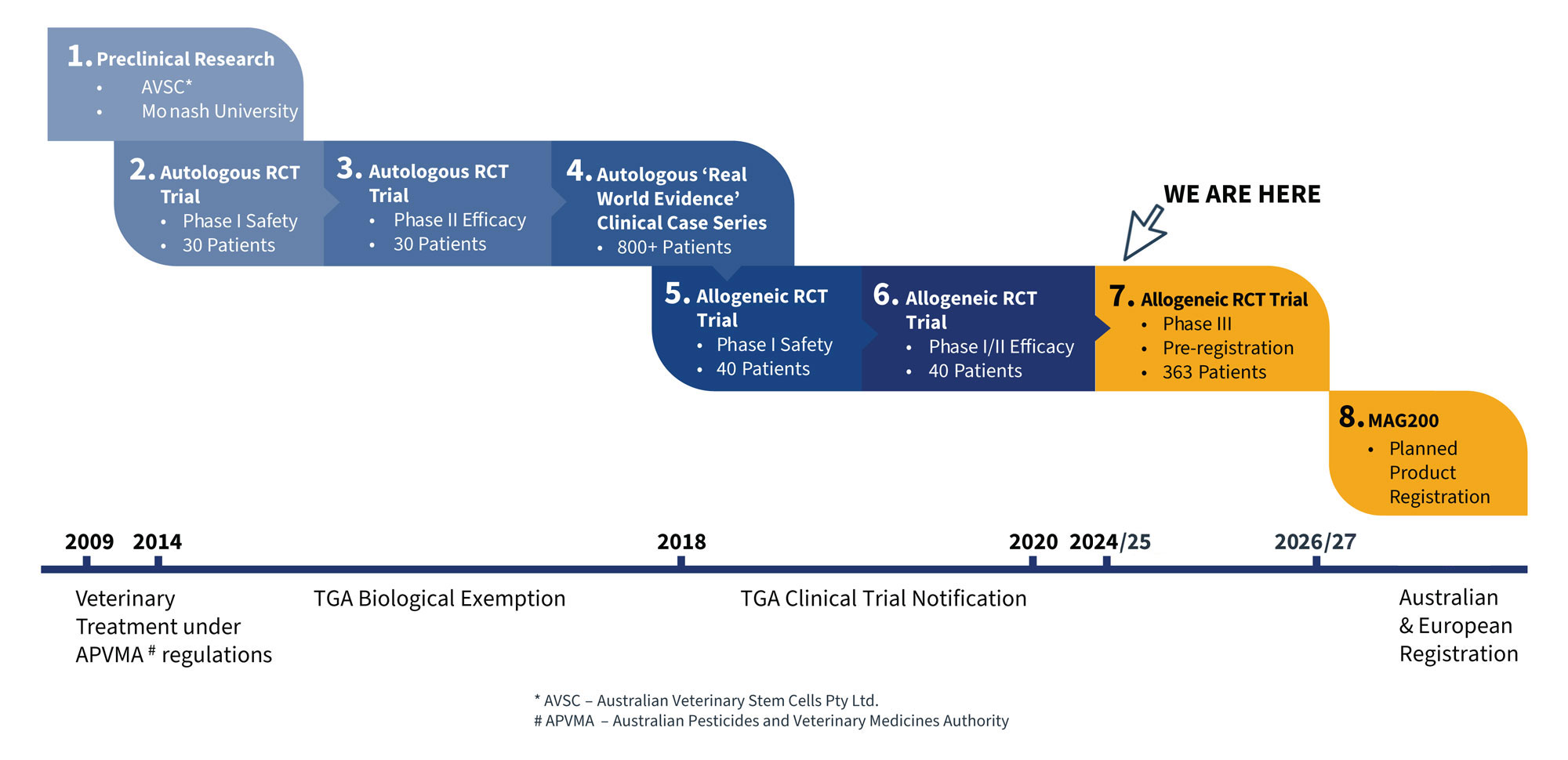
Magellan Stem Cells product MAG200 is an exciting investment opportunity for strategic investors, addressing a global and currently unmet medical need. Our 13 years of intensive research which includes pre-clinical research and Phase 1-2 trials for clinical safety and efficacy, our product MAG200 has been developed as an off-the-shelf allogeneic stem cell treatment for osteoarthritis.
In the first quarter of 2025, Magellan Stem Cells product MAG200 will enter a pivotal phase three clinical trial. Our trial synopsis has been reviewed, adjusted and accepted by the TGA and other international regulatory bodies (EMA, MHRA, FDA). The commencement of this world first phase three trial with a proven allogeneic stem cell line presents a prime opportunity for investors who value emerging technologies in biomedical research.
With unique proprietary information and intellectual property, supported by strong patent portfolio protection, Magellan Stem Cells product MAG200 is a scalable global treatment with the potential for significant market penetration.
Investment Opportunity
Magellan Stem Cells is looking for like-minded investment partners
We are in strategic discussions with interested investors and offering a minority interest in Magellan Stem Cells. We are looking for significant investment to help support the P3 clinical trial.
- There is a very large potential international market for an efficacious treatment of osteoarthritis
- More than 100 million people have in Europe, UK, USA, Canada, Japan and Australia are suffering from osteoarthritis
- The estimated cost of an approved product MAG200 treatment is less than 20% of the cost of joint replacement
- Magellan Stem Cells is expecting to generate substantial and increasing revenues from 2028 onwards and is projecting a positive EBITDA in the first year of commercial sales.
Magellan Stem Cells is expecting to generate substantial and increasing revenues from 2028 onwards and is projecting a positive EBITDA in the first year of commercial sales.
Board of Directors

Lou Panaccio
Non-Executive Chairman
Mr Panaccio is a Chartered Accountant with a wealth of executive management experience in business and healthcare services. Mr Panaccio has been the Chief Executive Officer of Monash IVF and Executive Director of Melbourne Pathology. He was also a Non-executive Director of ASX-listed Genera Biosystems Limited.
Mr Panaccio currently holds positions on the boards of ASX and NASDAQ listed Avita Therapeutics Inc. ASX50 company Sonic Healthcare Limited, ASX-listed Rhythm Non-executive Director.

Peter Hansen

Ross Williams

Michael Kenihan

Julien Freitag
Management Team

Kiran Shah

Julien Freitag

Richard Price

Renee Castelluccio
Next Steps
We welcome your interest and enquiries in investing in Magellan Stem Cells and its product MAG200 in preparation for P3 trial. Following is an outline of the steps involved to secure your investment.
Complete Non-Disclosure Agreement
Supply and review of Magellan’s investment information
Confirm Expression of Interest and Key Terms
Invitation to Magellan Data room
Detailed Q&A including medical and scientific matters
Review Legal Documentation
Complete Negotiations and Finalise Agreements
Execute Capital Transaction